Introduction: Non-persistence to treatment with hypomethylating agents (HMA) in higher-risk myelodysplastic syndrome (HR-MDS) patients can result in loss of response or ability to achieve a primary response. HMA treatment is recommended to be given to HR-MDS patients for least 4-6 treatment cycles to elicit a clinical response and premature termination is likely to result in poor outcomes and considerable healthcare spending. The study objective was to assess direct medical costs associated with HMA treatment non-persistence among HR-MDS patients.
Methods: Using a retrospective cohort design, MDS patients with refractory anemia with excess blasts (RAEB) were analyzed using 2010-2016 Surveillance, Epidemiology and End Results-Medicare linked database. RAEB diagnosis is considered to substantially overlap with HR-MDS and has been used as a surrogate for HR-MDS. The study cohort included RAEB patients diagnosed between 01/2011 and 12/2015. Patients were included if they had ≥ 1 year of continuous Medicare enrollment prior to diagnosis and did not receive stem cell transplant or lenalidomide in the follow-up period. HR-MDS patients receiving HMAs were stratified into HMA persistent (receiving 4 or more HMA cycles without any gap of ≥90 days between cycles) and HMA non-persistent (receiving less than 4 cycles or a gap of ≥90 days between cycles) groups. Healthcare resource use (HCRU) and associated direct medical costs incurred during the follow-up period were described as per-patient-per-month (PPPM). To account for baseline differences between HMA persistent and non-persistent groups, propensity score-based inverse probability of treatment weights (IPTW) were calculated. Weighted HCRU and costs (PPPM) were further estimated using generalized linear models (GLMs). Costs were inflated to 2019 USD using medical component of consumer price index.
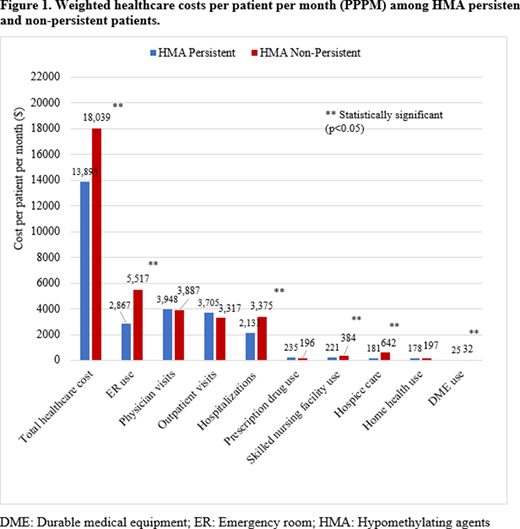
Results: There were 664 patients identified with RAEB, of which 295 (44.4%) patients were classified in the HMA non-persistent group and 369 (55.6%) patients in the HMA persistent group. HMA persistent and non-persistent groups were similar in baseline demographic and clinical characteristics; however, non-persistent HMA users were older at diagnosis and a lower proportion of patients were married. Results from weighted GLM analysis indicated higher PPPM resource utilization in HMA non-persistent patients compared to HMA persistent patients specifically for hospitalizations (Incident rate ratio [IRR], 1.543, 95% Confidence interval [CI]: 1.181 - 2.018]), ER visits (IRR= 1.322, 95% CI: 1.146 - 1.524), SNF use (IRR = 2.158, 95% CI: 1.308 - 3.560), home health (IRR = 1.335, 95% CI: 1.039 - 1.714] and hospice care use (IRR = 2.555, 95% CI:1.972 - 3.309) (Table 1). Further, HMA non-persistent patients had significantly (P<0.05) higher total PPPM costs than HMA persistent patients ($18,039 vs. $13,893, p<0.05); particularly for hospitalizations ($3,375 vs. $2,131), and ER costs ($5,517 vs. $2,867) (Figure 1).
Conclusions: A significant proportion of HR-MDS (RAEB) patients discontinue guideline-recommended HMA treatment. Non-persistence with HMA treatments was associated with substantial cost burden. The study findings call for closer care management by healthcare providers to ensure HMA treatment is completed as scheduled (unless directed otherwise by the provider) to manage outcomes and healthcare spending.
Joshi:Pharmerit International: Current Employment. Kale:Pharmerit International: Current Employment. Corman:Pharmerit International: Current Employment. Hill:Taiho Oncology: Current Employment, Current equity holder in publicly-traded company. Wert:Taiho Oncology: Current Employment, Current equity holder in publicly-traded company. Zeidan:Otsuka: Consultancy, Honoraria; Aprea: Research Funding; ADC Therapeutics: Research Funding; Leukemia and Lymphoma Society: Other; Epizyme: Consultancy, Honoraria; Ionis: Consultancy, Honoraria; Incyte: Consultancy, Honoraria, Research Funding; Jazz: Consultancy, Honoraria; Celgene / BMS: Consultancy, Honoraria, Research Funding; Astex: Research Funding; CCITLA: Other; Takeda: Consultancy, Honoraria, Research Funding; Cardiff Oncology: Consultancy, Honoraria, Other; BeyondSpring: Consultancy, Honoraria; Acceleron: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Boehringer-Ingelheim: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; MedImmune/Astrazeneca: Research Funding; Trovagene: Consultancy, Honoraria, Research Funding; Agios: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Taiho: Consultancy, Honoraria; Cardinal Health: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.